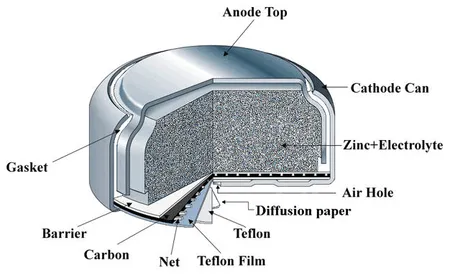
Zinc-Air Batteries
Zinc-air batteries are batteries characterised by their use of zinc and a reaction with atmospheric oxygen. There is both non-rechargeable (primary) and rechargeable (secondary) zinc-air batteries, although the primary batteries are far more common.
Chemistry
A zinc-air battery is a specific type of metal-air battery.
The reactions at each part of the battery are shown below:
Anode
This has a voltage potential of .
Fluid
Cathode
This has a voltage potential of .
Overall
This has a voltage potential of .
Voltage
The theoretical voltage of a zinc-air cell is 1.65V (based on the chemistry). In reality, the voltage of a zinc-air cell is between 1.35-1.4V.
Energy Density
One of zinc-air’s unique properties is that they consume atmospheric oxygen as part of the chemical reaction. This means that they can have higher energy densities than other similar batteries because one of the reactants is not actually contained within the battery.
Storage
Zinc-air batteries can be stored without losing much energy as long as oxygen is kept away from the battery. Digital hearing aids come with a little tab that must be removed before use, this tab prevents air from entering the battery and reacting with the zinc. They can last for 3 years with little capacity loss.
Applications
Most digital hearing aids use zinc-air button cells. You remove a tab to allow air into the battery, starting the reaction that produces an electro-motive force (i.e. the voltage).